|
Author list |
Singh, Jatinder; Kim, Dong Hwan; Kim, Eun-Hee; Kim, Hyunuk; Hadiputra, Rizky; Jung, Jaehoon; Chi, Ki-Whan |
|
Abstract |
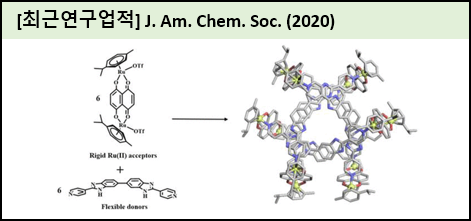
Engineering of supramolecular topologies offers potential opportunities for tailoring their properties to various function and applications. However, the synthesis of interlocked or intertwined compounds, catenanes, links or knots, is a challenge. Previously, we used coordination-driven self-assembly and noncovalent interactions (NCIs) between metal-based acceptors and multipyridyl donors to create supramolecular topologies with increasing complexity. Self-assembling components of fixed length and geometry have been utilized for the production of topologies such as Borromean rings, Solomon links, Hopf's link, "rectangle in rectangle", and an 818 molecular knot. However, recent synthesis of a linear [3]catenane by us witnessed the importance of flexible ligands along with coordination-driven self-assembly and NCIs in self-assembling units. This flexibility provides distinctive angularity for the recognition of various NCIs and thus offers tremendous possibilities for realizing complex supramolecular topologies. This study proposed a selective and quantitative synthesis, and also the first X-ray characterization of a closed three-link chain (a prime link of [3]catenane with 6 crossings) via two component coordination-driven self-assembly. The experiments based upon concentration, guest template, and solvent effects were systematically presented. Furthermore, the experimental finding was supported by density functional theory calculations, which highlighted the necessity of the multiple NCIs along with appropriate geometry of the [2 + 2] rings.
|