|
Author list |
Yuvaraj Subramanian, Rajesh Rajagopal , Baskar Senthilkumar, Yong Joon Park, Sung Kang, Yu Jin Jung, Kwang-Sun Ryu* |
|
Abstract |
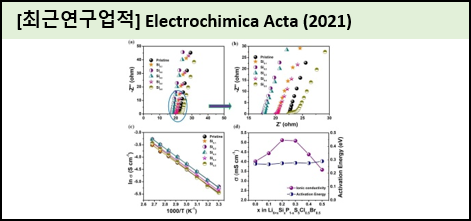
Lithium solid state batteries are one of the state of the art energy storage systems due to their high safety. However, ionic conductivity in solid electrolytes is a concern, because at present it does not match the ionic conductivity of non-aqueous Li-ion batteries, thus resulting in sluggish electrochemical kinetics. In this report, we enhance the ionic conductivity of Li-argyrodites (Li6PS5Cl0.5Br0.5) through Si substitution at the P-site using a dry ball milling process. Among the silicon substitutions, Li6.2Si0.2P0.8S5Cl0.5Br0.5 exhibited the high ionic conductivity of 5.12 mS cm?1 compared to pristine Li6PS5Cl0.5Br0.5 at 4.02 mS cm?1. The Rietveld refinement analysis revealed that after silicon substitution, volume of the unit cell gets increased that allows the lithium at T2-site, that promotes the fast Li-ion transport. Moreover, the optimized solid electrolyte was utilized in a solid state battery system, and demonstrated a high initial capacity of 148.1 mAh g?1 at 0.1 C rate compared to pristine argyrodite (135.1 mAh g?1). Further, we demonstrated the interface phenomena between electrode and solid electrolyte using ex-situ XPS analysis. This confirmed the formation of interface products such as LiCl, Li2S, lithium polysulfides and P2Sx, which influence the cycling stability of the ASSLBs. |