|
Abstract |
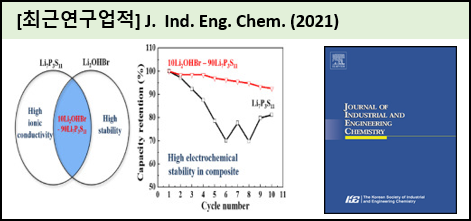
Li7P3S11 solid electrolytes with high lithium-ion conductivity are promising candidates for use in all-solid-state lithium batteries. However, this electrolyte's poor interfacial compatibility with lithium electrodes causes unstable cyclability. In this study, in order to address this problem, (100-x)Li7P3S11-xLi2OHBr (xㅤ=ㅤ0, 2, 5, 10, 20, 30, 40, and 50) electrolytes are prepared by a high energy ball-milling technique and heat-treatment process. The resulting (100-x)Li7P3S11-xLi2OHBr (xㅤ=ㅤ2, 5, 10, 20, 30, 40, and 50) electrolytes provide improved electrochemical performance with good cycling stability and a wide electrochemical window of up to 10V (vs. Li/Li+). Moreover, these electrolytes have high ionic conductivity of 10^(-4)-10^(-4) S/cm at room temperature. Particularly, the 90Li7P3S11-10Li2OHBr electrolyte displays the highest conductivity of 4.4 *10^(-4) S/cm at room temperature as well as improved cyclability. Moreover, 90Li7P3S11-10Li2OHBr shows decreased interfacial resistance between the solid electrolyte and cathode electrode, which was revealed by Electrochemical Impedance Spectroscopy (EIS) analysis. The initial discharge capacity of 90Li7P3S11-10Li2OHBr was found to be 135 mAh/g when used in a In|solid electrolyte|Li(Ni0.6Co0.2Mn0.2)O2 all-solid-state lithium battery (ASSLB). Thus, we can conclude the addition of Li2OHBr into the Li7P3S11 results in enhanced electrochemical properties. |