|
Abstract |
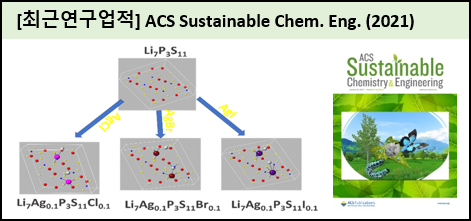
A new silver halide-doped Li7P3S11 (7Li2S-3P2S5) solid electrolyte with increasing ionic conductivity and stability was prepared by the wet ball milling process. Detailed structural investigations were carried out after the doping process. First, AgI was added to a Li7P3S11 solid electrolyte to achieve a novel composite/phase. The highest ionic conductivity of the composite was optimized by changing the AgI concentration (0.05, 0.10, and 0.25) and the sintering temperature (240, 270, and 300 캜). The selected Li7Ag0.1P3S11I0.1 solid electrolyte displayed a high ionic conductivity of 1.35 ㅠ 10-3 S cm-1 at room temperature, which is higher than the prepared pristine Li7P3S11 solid electrolyte. High-power X-ray diffraction analysis is used to analyze the crystalline nature of the electrolyte. The surface morphology of the electrolyte was studied by the field-emission microscopy technique. The surface functional group of the Li7Ag0.1P3S11I0.1 solid electrolyte was analyzed by the X-ray photoelectron spectroscopy technique. The electrochemical behavior of the selected electrolyte was analyzed by cyclic voltammetry, DC polarization, and impedance spectroscopy techniques. Cyclic voltammetry and DC polarization showed that the synthesized Li7Ag0.1P3S11I0.1 solid electrolyte is highly stable up to 5 V potential window and has charge-discharge stability of more than 100 cycles against Li metal. AgCl- and AgBr-doped Li7P3S11 solid electrolytes (Li7Ag0.1P3S11Cl0.1 and Li7Ag0.1P3S11Br0.1) were also prepared and their structural and electrochemical properties were studied. Thus, AgX-doped Li7P3S11 solid electrolyte exhibited improved ionic conductivity and air stability than the prepared pristine Li7P3S11 solid electrolyte. |