|
Title |
Catalytic transfer hydrogenation of furfural to furfuryl alcohol under mild conditions over Zr-MOFs: exploring the role of metal node coordination and modification |
|
Author list |
Valekar, Anil H; Lee, Minhui; Yoon, Ji Woong; Kwak, Jaesung; Hong, Do-Young; Oh, Kyung-Ryul; Cha, Ga-Young; Kwon, Young-Uk; Jung, Jaehoon; Chang, Jong-San; Hwang, Young Kyu |
|
Publication date |
2020/03 |
|
Citation information |
ACS Catalysis, 10, 3720 (2020)
|
|
DOI |
10.1021/acscatal.9b05085 |
|
Graphical Abstract |
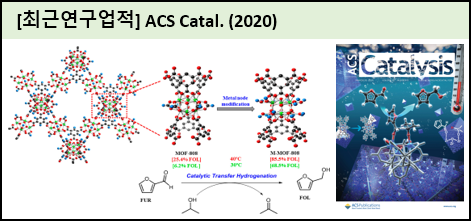
|
|
Abstract |
The catalytic transfer hydrogenation (CTH) reaction is considered as a potential route for upgrading bio-based carbonyls to their corresponding alcohols. Herein, a series of Zr-based metal?rganic frameworks (Zr-MOFs) containing various types of metal node to ligand coordinations were synthesized and tested for CTH of furfural (FUR) to furfuryl alcohol (FOL). It was found that metal node coordination plays a more important role than porosity in Zr-MOFs. MOF-808 (synthesized using a scaled-up approach to achieve a higher batch yield), with the lowest metal node to ligand coordination (coordination number 6), was found to be the most active catalyst among the various tested Zr-MOFs. Furthermore, M-MOF-808, modified by simple methanol activation (M), outperformed the pristine MOF-808 in CTH of FUR to FOL even at 30 ℃ in the presence of 2-propanol (IPA) as the hydrogen source. The simple modification of the metal node in the Zr-MOF changed the acid-base properties of the MOF-808 surface through the development of coordinatively unsaturated sites (CUS), hydroxyl and methoxy groups in the framework of the Zr-MOF, which probably help to facilitate the adsorption of FUR and IPA onto the metal node surfaces of the catalyst. To evaluate the versatility of methanol activation in CTH, further substrates, including other types of biomass and representative carbonyl compounds over M-MOF-808, were tested. To demonstrate heterogeneous catalysis, the catalyst was recycled for five consecutive cycles, with little loss after the first cycle, and was fully characterized to observe any changes in its structure. Mechanistic insights were provided by isotopically labeled 2-propanol-d8 experiments, indicating FUR reduction through transfer hydrogenation. Finally, the reaction mechanism for CTH of FUR to FOL was proposed in detail using density functional theory (DFT) calculations over metal node modified model systems of a 6-connected Zr-MOF. | |

