|
Abstract
(superscript and subscript cannot be allowed.) |
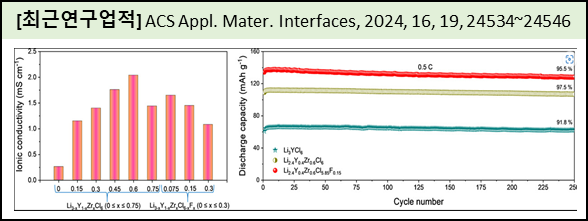
Recently, the halide solid electrolyte (SE) system has been widely used in lithium solid-state batteries due to their specific properties, such as the high electrochemical stability window that prevents any side reaction with the electrode/electrolyte interface. Conspicuously, the halide SE possesses very low ionic conductivity values in the range (0.2–0.5) mS cm–1. In this work, we enhance the ionic conductivity of Li3YCl6 SE by the substitution of low-cost Fe and Zr elements on the Y-site and F on the Cl site, in which the electrolyte is prepared through high-energy ball milling without a heat treatment process. The structural analysis reveals that the prepared halide SEs showed the pure phase of the Li3YCl6 tetragonal crystal structure and were free from impurity phases. In the prepared composition, the Li2.4Y0.4Zr0.6Cl6 and Li2.4Y0.4Zr0.6Cl5.85F0.15 electrolyte exhibited a higher ionic conductivity of 2.05 and 1.45 mS cm–1, respectively, than Li3YCl6 (0.26 mS cm–1). Interestingly, the Li2.4Y0.4Zr0.6Cl5.85F0.15 electrolyte possesses a better electrochemical stability window of 1.29–3.9 V than Li2.4Y0.4Zr0.6Cl6 (2.1–3.79 V). Moreover, the electrochemical results revealed that the assembled solid-state battery using Li2.4Y0.4Zr0.6Cl6 and Li2.4Y0.4Zr0.6Cl5.85F0.15 electrolyte demonstrated the higher initial Coulombic efficiency of 84.7 and 87%, respectively, than Li3YCl6 of 82.6%. We consider Li2.4Y0.4Zr0.6Cl5.85F0.15 to be an important electrolyte candidate in all-solid-state batteries. |