|
Abstract
(superscript and subscript cannot be allowed.) |
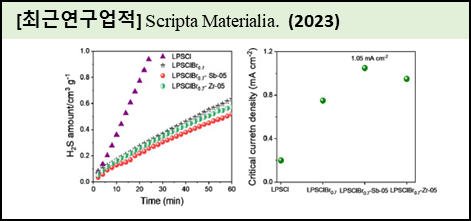
Sulfide solid electrolyte has attracted much interest in the field of lithium solid-state batteries due to its high ionic conducting nature and good compatibility with lithium metal. However, improving the ionic conductivity, air stability, and electrochemical compatibility of sulfide solid electrolytes remains elusive. In this work, the Li-argyrodite system was optimized through halogen (Br) and metal (Sn and Zr) substitution using a high-energy ball milling process followed by heat treatment. The ionic conductivity analysis revealed that the optimized composition of Li5.3PS4.3Cl1.0Br0.7 solid electrolyte demonstrates an ionic conductivity of 16.6 mS cm−1 at 30 °C, which was ∼3 fold higher than that of Li6PS5Cl electrolyte. The metal substitution in the optimized composition slightly reduced the ionic conductivity, but increased the air stability and lithium compatibility. The optimized Sb- substituted electrolyte utilized in the Li-battery delivered a high discharge capacity of 72 mAh g−1 at 1 C rate than other electrolytes. |