|
Author list |
Khoirul Umam, Byung Cheol Sin, Laxman Singh, Chaewon Moon, Jaeeun Choi, Inyoung Lee, Jaewoong Lim, Jaehoon Jung, Myoung Soo Lah, Youngil Lee |
|
Abstract |
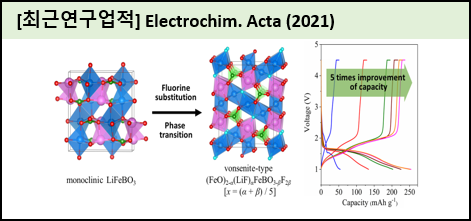
Among polyanion-type cathode materials used for large-scale lithium-ion batteries (LIBs), LiFeBO3 (LFeB) has received attention due to its lowest weight framework with a larger theoretical capacity of 220 mAh g?1 compared with commercialized LiFePO4 (170 mAh g?1). The main drawback of LFeB, however, is its poor specific discharge capacity as a cathode material for LIBs. Herein, the fluorine-substituted LFeB at the oxygen site, LiFeBO3-xF2x (LFeBF, x = 0.05, 0.1, 0.2, 0.3, and 0.5), has been prepared as a cathode material for LIBs via a solid-state reaction to improve the electrochemical behavior accompanied by phase transition. Morphological change as increasing x and well-distributed fluorine element of LFeBF have been observed using a scanning electron microscope combined with an energy dispersive X-ray spectrometer. X-ray diffraction, X-ray photoelectron spectroscopy, and solid?tate 7Li and 11B nuclear magnetic resonance spectroscopy studies of LFeBF as well as increasing x show a dramatic phase transition from monoclinic to vonsenite-type structure. The plausible atomic arrangement has been also investigated using density functional theory. Furthermore, the fluorine substitution at the oxygen site of LFeB leads to a remarkable improvement in discharge capacity, the highest value (361.15 mAh g-1 for LFeBF (xㅤ= 0.3)) of which is about five times larger than that of LFeB (73.43 mAh g-1) at 0.05 C rate, without any additional carbon source. |