|
Abstract |
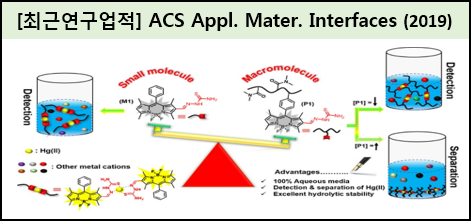
Developing a simple and cheap analytical method for the selective detection and quantitative separation of toxic ions present in aqueous media is the biggest challenge faced by the chemosensing research community. Here, a 5,5- difluoro-1,3,7,9-tetramethyl-10-phenyl-5H-dipyrrolo-diazaborinine-derived water-soluble polymer integrated with thiosemicarbazone units was rationally designed and synthesized for the simultaneous detection and separation of Hg(II) ions in pure aqueous solution. The water-soluble polymer scaffold poly(N,N′-dimethyl acrylamide-co-5,5-difluoro-1,3,7,9-tetramethyl-10-phenyl-5H-dipyrrolo-diazaborinine-2-carbaldehyde) was synthesized by reversible addition-ragmentation chain transfer polymerization, followed by post-polymerization modification with thiosemicarbazide, leading to the formation of the target probe, P1. The nonemitting P1 exhibited bright yellow emission upon exposure to Hg(II) ions, with a limit of detection as low as 0.37 iM. This turn-on emission behavior triggered by Hg(II) ions might originate from the suppression of isomerization around the C-N bond of the thiosemicarbazone moiety caused by the formation of a coordination complex between P1 and Hg(II) ions. In addition, P1 displayed excellent selectivity toward Hg(II) ions over other metal cations. Finally, the selective removal of Hg(II) ions from an aqueous solution containing various metal ions was achieved by precipitation, which is probably caused by the fact that coordination complexes whereby Hg(II) ions acted as bridgeheads between P1 molecules had formed.
|