|
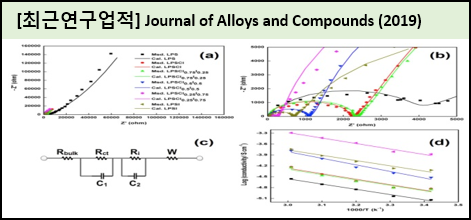
We prepared 3Li2S-P2S5 glass ceramic solid electrolyte co-doped with various concentrations of Cl and I via solution synthesis using acetonitrile as the solvent. The prepared glass ceramic electrolyte was characterized by various physiochemical techniques. The electrochemical stability and conductivity of the prepared 3Li2S-P2S5:xLiCl:(1-x)LiI GCS electrolyte was studied by cyclic voltammetry and electrochemical impedance spectroscopy. The electrical conductivity of 3Li2S-P2S5:xLiCl:(1-x)LiI GCS was higher than that of a pure 3Li2S-P2S5GCS electrolyte.
The 3Li2S-P2S5:xLiCl:(1-x)LiI solid electrolytes were prepared via solution process using acetonitrile, and crystallization was achieved by heating at 160℃ for 3h. The crystalline nature and morphology of the prepared materials were studied using powder X-ray diffraction and field emission scanning electron microscope analysis. Cyclic voltammetry was performed to confirm the electrochemical stability of the electrolytes. The ionic conductivity of the prepared 3Li2S-P2S5:0.25LiCl:0.75LiI solid electrolyte was 1.32 ×10Scm at room temperature. In addition, the prepared 3Li2S-P2S5:0.25LiCl:0.75LiI solid electrolyte showed better electrochemical stability comparable to that of prepared pure 3Li2S-P2S5, and other Cl and I mixed solid electrolytes.
|