|
Abstract
(superscript and subscript cannot be allowed.) |
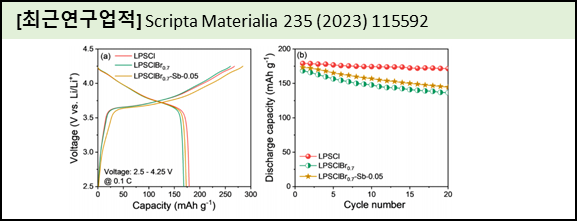
Sulfide solid electrolyte has recently come to be considered a suitable electrolyte for lithium batteries because of its high ionic conductivity. However, it cannot be implemented in real time applications because of lithium dendrites and moisture sensitive issues. In this work, we solve the aforementioned issues through Sc2O3 substitution on P and S-site in Li6PS5Cl structure. The prepared Sc2O3 substituted Li6PS5Cl shows an ionic conductivity values of 3.80 mS cm−1, which is somewhat lower compared to Li6PS5Cl (4.91 mS cm−1). Notably, after Sc2O3 substitution in Li6PS5Cl that boost up the interface stability with lithium. Moreover, it exhibits an excellent critical current density of 0.6 mA cm−2 than bare electrolyte (0.2 mA cm−2). Also, it shows the better DC cyclability at 0.2 and 0.3 mA cm−2 without internal short circuit after 200 cycles. Atmospheric stability analysis assures that the optimized electrolyte shows high tolerance against dry air. Finally, we assembled the battery that shows the discharge capacity of 183.3 mAh g−1, and the capacity is found to be retained over 94.5 % after 180 cycles. Importantly, the battery demonstrates a good discharge capacity of 139.9 mAh g−1 at 1C rate. |